Florida 3170 Form in PDF
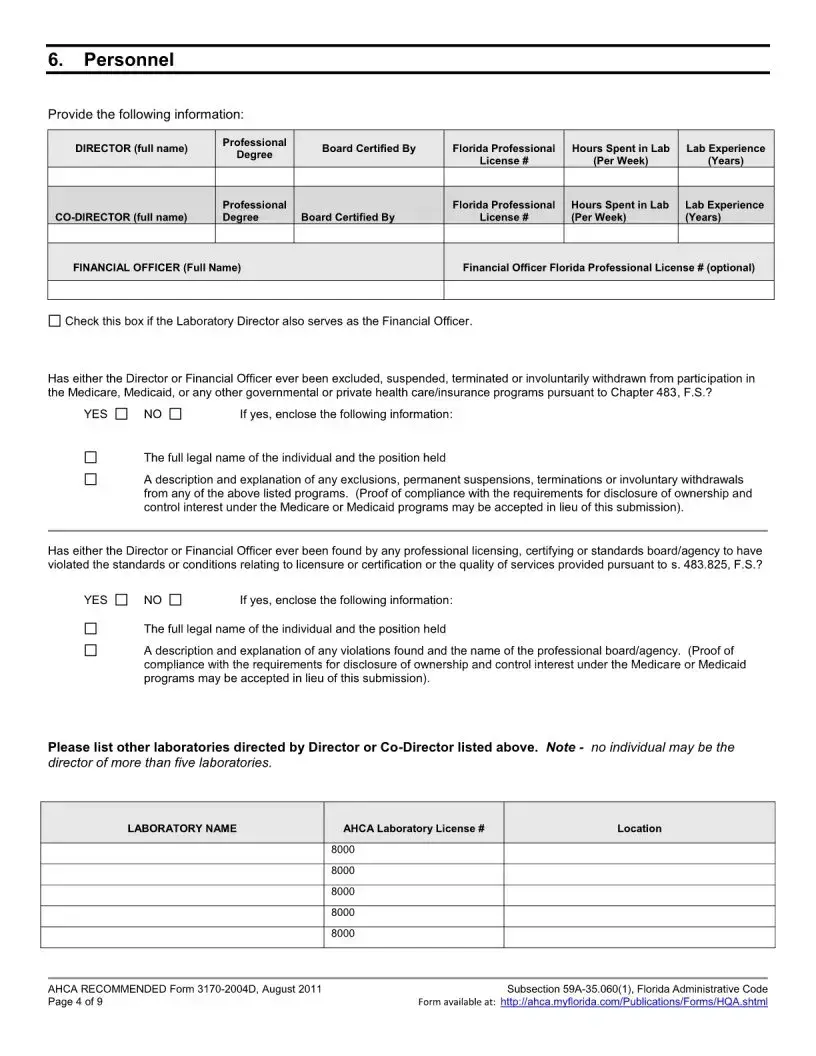
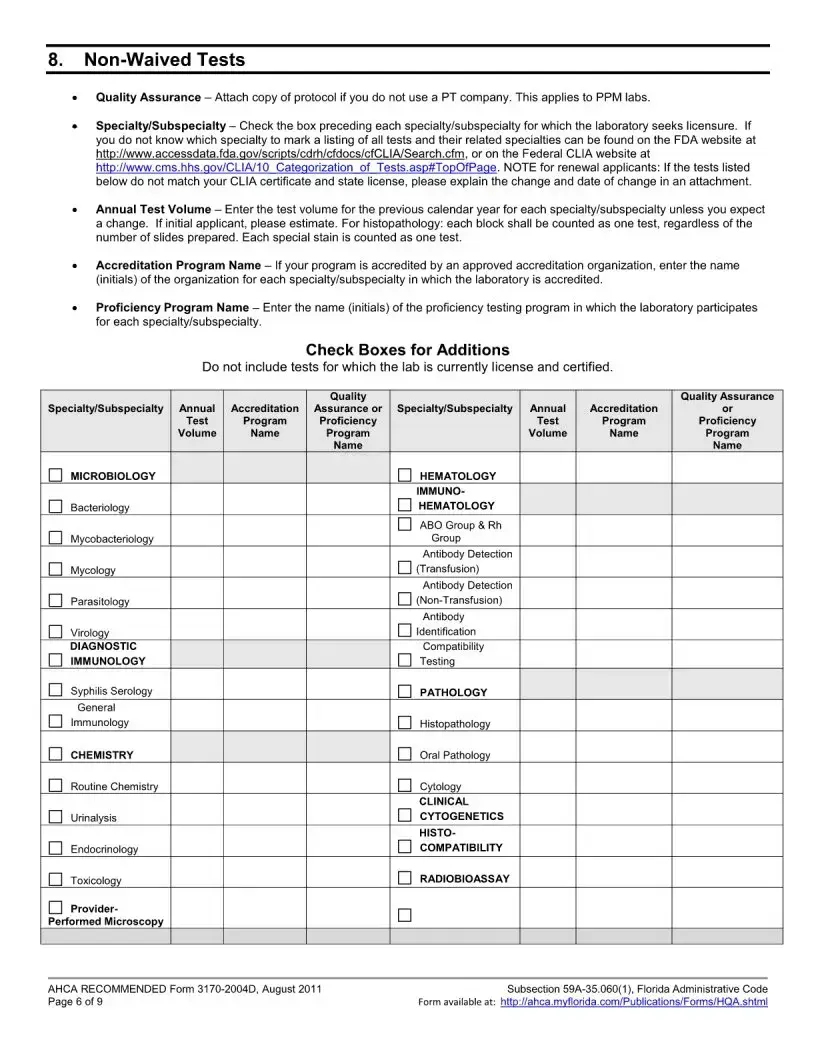
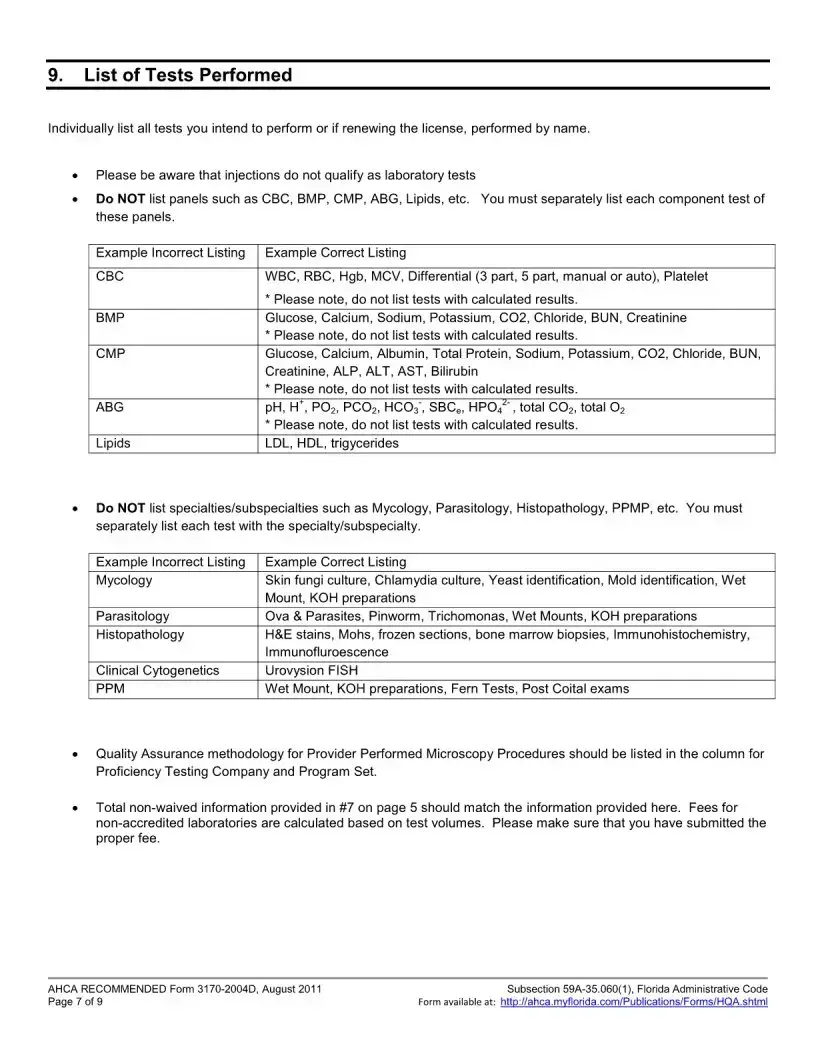
The Florida 3170 form is a Health Care Licensing Application specifically for Clinical Laboratories that are seeking to add a specialty, subspecialty, or make a change in specialty outside of the regular licensure renewal period. This form requires applicants to provide specific documentation and fees to ensure compliance with Florida health care regulations. Timely submission is crucial to avoid late fees and ensure the application is processed efficiently.
Customize Florida 3170 Here
Florida 3170 Form in PDF
Customize Florida 3170 Here
Customize Florida 3170 Here
or
⇩ Florida 3170 File
Don’t forget to finish your form
Edit and finish Florida 3170 online in just minutes.